RenovaCare, Inc., (OTCQB: RCAR), today highlighted an analysis of treatment results from 44 patients treated for severe second-degree wide-area burn injuries, as published in Burns, the peer-reviewed Journal of the International Society for Burn Injuries. The treatment method, which involved isolating and spraying the patient’s own skin stem cells on to burn wounds, is the technology underlying the RenovaCare patented SkinGun™* and CellMist™ System*.
This press release features multimedia. View the full release here: http://www.businesswire.com/news/home/20180130005343/en/
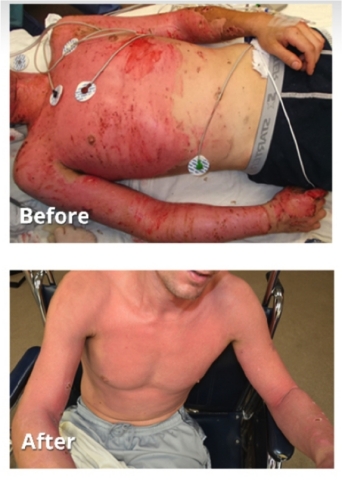
Burn patients treated with technology behind RenovaCare SkinGun(TM). Source: Burns, Journal of the International Society for Burn Injuries.
The results, published in the November 2017 issue of Burns, report the retrospective analysis of outcomes in 44 severe second-degree burn patients who received skin stem cell spray grafting treatment under an innovative practice approach.
According to the Burns article, spraying deep partial-thickness burn wounds with a patient’s own skin stem cells “...can achieve rapid wound re-epithelialization, particularly in large wounds.” Re-epithelialization marks the final stage of successful healing and is an essential component of wound closure. At this stage, skin function is restored and the risk of infection is eliminated.
“Treated patients presented with a variety of burn wound etiologies and a wide range of TBSA (Total Burn Surface Area). Overall clinical results were very satisfying,” stated the authors.
Regardless of the severity or size of the wound, the average days of healing and discharge remained low. “The mean days to [hospital] discharge was 6.28 +/- 4.1 with a median of six days,” according to the study.
“This data is especially compelling because patients were successfully treated for a broad spectrum of burn injuries and vast burn surface areas. Importantly, patients had very short hospital stays, which, we believe could deliver a better healing experience for patients and lower costs for hospitals and insurance providers,” stated Mr. Thomas Bold, President and CEO of RenovaCare, Inc.
“The clinical outcomes and minimal hospital stays for severe burn patients published in this article supports our conviction to bring our SkinGun™ technology to market as quickly as possible,” continued Mr. Bold. “Presently, we’re finalizing the requisite documentation for filing with the FDA to demonstrate the safety of our approach for treating wounds using a patient’s own skin cells.”
The retrospective analysis covered 44 patients treated for deep partial-thickness burns, including gas and chemical explosions, and electrical, gasoline, hot water and tar scalding burns. Patients were treated for burns as large as 7.53 square feet (7,000 cm2). The majority of burns were between 1.2 to 3.2 square feet (1,000 - 3,000 cm2).
The Burns article titled, “Cell-spray auto-grafting technology for deep partial thickness burns: Problems and solutions during clinical implementation,” by: Roger Esteban-Vives, Alain Corcos, Myung S. Choi, Matthew T. Young, Patrick Over, Jenny Ziembicki, Jörg C. Gerlach, was published by Elsevier in the November 2017 issue of Burns 2017 Nov 25. pii: S0305-4179(17)30558-2. doi: http://dx.doi.org/10.1016/j.burns.2017.10.008. Epub 2017 Nov 25).
A previously published Burns article titled, “Second-degree burns with six etiologies treated with autologous noncultured cell-spray grafting”, by: Roger Esteban-Vives, Myung S. Choi, Matthew T. Young, Patrick Over, Jenny Ziembicki, Alain Corcos, and Jörg Gerlach, was published by Elsevier in the November 2016 issue of Burns (Nov;42(7):e99-e106. doi: 10.1016/j.burns.2016.02.020. Epub 2016 Aug 25).
Study authors, Dr. Roger Esteban-Vives and Dr. Jörg Gerlach currently have a financial interest in the SkinGun™ spray-grafting technology through payments from RenovaCare, Inc. Dr. Esteban-Vives, who currently Director of Cell Sciences at RenovaCare, Inc., was a postdoctoral fellow at the University of Pittsburgh when this work was conducted and did not have such financial interest at that time.
*RenovaCare products are currently in development and are not available for sale in the United States. There is no assurance that the company’s planned or filed submissions to the U.S. Food and Drug Administration, if any, will be accepted or cleared by the FDA.
About Burns
Burns aims to foster the exchange of information among all engaged in preventing and treating the effects of burns. The journal focuses on clinical, scientific and social aspects of these injuries and covers the prevention of the injury, the epidemiology of such injuries and all aspects of treatment including development of new techniques and technologies and verification of existing ones. Regular features include clinical and scientific papers, state of the art reviews and descriptions of burn-care in practice.
About RenovaCare
RenovaCare, Inc. is developing first-of-their-kind autologous (self-donated) stem cell therapies for the regeneration of human organs, and novel medical grade liquid sprayer devices. RenovaCare, Inc. is developing first-of-its-kind autologous (self-donated) stem cell therapies for the regeneration of human organs. Its initial product under development targets the body’s largest organ, the skin. The company’s flagship technology, the CellMist™ System, uses its patented SkinGun™ to spray a liquid suspension of a patient’s stem cells – the CellMist™ Solution – onto wounds. RenovaCare is developing its CellMist™ System as a promising new alternative for patients suffering from burns, chronic and acute wounds, and scars. In the US alone, this $45 billion market is greater than the spending on high-blood pressure management, cholesterol treatments, and back pain therapeutics.
For additional information, please call Drew Danielson at: 888-398-0202 or visit: https://renovacareinc.com
To receive future press releases via email, please visit: https://renovacareinc.com/investors/register/
Follow us on Twitter https://twitter.com/RenovaCareInc or follow us on Facebook https://www.facebook.com/renovacarercar
For answers to frequently asked questions, please visit our FAQ’s page: https://renovacareinc.com/investors/faqs/
Social Media Disclaimer
Investors and others should note that we announce material financial information to our investors using SEC filings and press releases. We use our website and social media to communicate with our subscribers, shareholders, and the public about the company, RenovaCare, Inc. development, and other corporate matters that are in the public domain. At this time, the company will not post information on social media that could be deemed to be material information unless that information was distributed to public distribution channels first. We encourage investors, the media, and others interested in the company to review the information we post on the company’s website and the social media channels listed below:
* This list may be updated from time to time.
Legal Notice Regarding Forward-Looking Statements
No statement herein should be considered an offer or a solicitation of an offer for the purchase or sale of any securities. This release contains forward-looking statements that are based upon current expectations or beliefs, as well as a number of assumptions about future events. Although RenovaCare, Inc. (the “Company”) believes that the expectations reflected in the forward-looking statements and the assumptions upon which they are based are reasonable, it can give no assurance that such expectations and assumptions will prove to have been correct. Forward-looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “could,” “expect,” “anticipate,” “estimate,” “believe,” “intend,” or “project” or the negative of these words or other variations on these words or comparable terminology. The reader is cautioned not to put undue reliance on these forward-looking statements, as these statements are subject to numerous factors and uncertainties, including but not limited to: the timing and success of clinical and preclinical studies of product candidates, the potential timing and success of the Company’s product programs through their individual product development and regulatory approval processes, adverse economic conditions, intense competition, lack of meaningful research results, entry of new competitors and products, inadequate capital, unexpected costs and operating deficits, increases in general and administrative costs, termination of contracts or agreements, obsolescence of the Company's technologies, technical problems with the Company's research, price increases for supplies and components, litigation and administrative proceedings involving the Company, the possible acquisition of new businesses or technologies that result in operating losses or that do not perform as anticipated, unanticipated losses, the possible fluctuation and volatility of the Company's operating results, financial condition and stock price, losses incurred in litigating and settling cases, dilution in the Company's ownership of its business, adverse publicity and news coverage, inability to carry out research, development and commercialization plans, loss or retirement of key executives and research scientists, and other risks. There can be no assurance that further research and development will validate and support the results of our preliminary research and studies. Further, there can be no assurance that the necessary regulatory approvals will be obtained or that the Company will be able to develop commercially viable products on the basis of its technologies. In addition, other factors that could cause actual results to differ materially are discussed in the Company's most recent Form 10-Q and Form 10-K filings with the Securities and Exchange Commission. These reports and filings may be inspected and copied at the Public Reference Room maintained by the U.S. Securities & Exchange Commission at 100 F Street, N.E., Washington, D.C. 20549. You can obtain information about operation of the Public Reference Room by calling the U.S. Securities & Exchange Commission at 1-800-SEC-0330. The U.S. Securities & Exchange Commission also maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the U.S. Securities & Exchange Commission at http://www.sec.gov. The Company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
View source version on businesswire.com: http://www.businesswire.com/news/home/20180130005343/en/


























